Acute Myeloid Leukemia Clinical Trial Pipeline Appears Robust With 100+ Key Pharma Companies Actively Working in the Domain | DelveInsight
Acute myeloid leukemia (AML) is an aggressive cancer of the blood and bone marrow, characterized by rapid growth of abnormal white blood cells. As AML primarily affects older adults, the global rise in aging populations is significantly increasing the at-risk patient base. This demographic shift is driving higher diagnosis rates and intensifying demand for more effective, better-tolerated treatments. Therefore, the market is seeing strong momentum in targeted therapies, immunotherapies, and personalized treatment approaches.
New York, USA, Dec. 04, 2025 (GLOBE NEWSWIRE) -- Acute Myeloid Leukemia Clinical Trial Pipeline Appears Robust With 100+ Key Pharma Companies Actively Working in the Domain | DelveInsight
Acute myeloid leukemia (AML) is an aggressive cancer of the blood and bone marrow, characterized by rapid growth of abnormal white blood cells. As AML primarily affects older adults, the global rise in aging populations is significantly increasing the at-risk patient base. This demographic shift is driving higher diagnosis rates and intensifying demand for more effective, better-tolerated treatments. Therefore, the market is seeing strong momentum in targeted therapies, immunotherapies, and personalized treatment approaches.
DelveInsight’s 'Acute Myeloid Leukemia Pipeline Insight 2025' report provides comprehensive global coverage of pipeline therapies for acute myeloid leukemia across various stages of clinical development. The report offers an in-depth analysis of key trends, emerging therapies, and competitive landscape dynamics, highlighting the strategies of major pharmaceutical companies to advance the pipeline and capitalize on future growth opportunities. In addition, it includes critical insights into clinical trial benchmarking, partnering and licensing activities, and regulatory pathways involving the FDA and EMA, enabling stakeholders to make informed decisions and optimize development strategies within the acute myeloid leukemia domain.
Acute Myeloid Leukemia Clinical Trial Analysis Summary
- DelveInsight’s acute myeloid leukemia pipeline report depicts a robust space with 100+ active players working to develop 150+ pipeline acute myeloid leukemia drugs.
- Key acute myeloid leukemia companies such as HUTCHMED, Senti Biosciences, Shijiazhuang Yiling Pharmaceutical Co. Ltd, Apollo Therapeutics, Immune-Onc Therapeutics, Molecular Partners, Kling Biotherapeutics, Chordia Therapeutics, Inc., Cullinan Therapeutics Inc., Moleculin Biotech, Inc., Advesya, AstraZeneca, Akeso, Galecto Biotech, TC Biopharm, Hangzhou Polymed Biopharmaceuticals, Inc., Ascentage Pharma, Ryvu Therapeutics, Guangzhou Bio-gene Technology Co., Ltd, CSPC ZhongQi Pharmaceutical Technology Co., Ltd., Remedy Plan, Inc., Advanced BioDesign, Ellipses Pharma, CCM Biosciences, HiberCell, Inc. and others are evaluating new acute myeloid leukemia drugs to improve the treatment landscape.
- Promising pipeline acute myeloid leukemia therapies, such as HMPL-306, SENTI 202, XY0206, APL 4098, IO 202, MP0533, KBA 1331, CTX-712, CLN-049, Annamycin, CCTx-001, AZD3632, AK117, GB3226, TCB008, HPB-092, APG-2575, RVU120, BG1805, SYHX1903, RPT1G, ABD-3001, EP0042, CCM-445, HC-7366 and others, are in different phases of Acute Myeloid Leukemia clinical trials.
- Approximately 10+ acute myeloid leukemia drugs are in the late stage of development, whereas 50+ drugs are in the mid and early stages of development.
- Notable MoAs in acute myeloid leukemia clinical trials include Isocitrate dehydrogenase 1 inhibitors; Isocitrate dehydrogenase 2 inhibitors, Type II DNA topoisomerase inhibitors, Fms-like tyrosine kinase 3 inhibitors, LILRB4 protein inhibitors, CLK kinase inhibitors, GCN2 Inhibitor, GCN2 Modulator, Antibody-dependent cell cytotoxicity, SNRNP200 protein modulators, Interleukin-1 receptor accessory protein inhibitors, CD47 antigen inhibitors and others.
Request a sample and find out what is the latest treatment for acute myeloid leukemia @ Acute Myeloid Leukemia Pipeline Report
What is Acute Myeloid Leukemia?
Acute myeloid leukemia (AML) is a cancer that impacts the bone marrow and blood. In this condition, the bone marrow produces abnormal myeloid cells, immature white blood cells that would normally mature into red blood cells, white blood cells, and platelets. These abnormal cells multiply quickly, crowding out healthy blood cells and disrupting normal bone marrow function. As a result, the body experiences a shortage of normal blood cells, leading to a range of symptoms and complications. Common signs and symptoms of AML include fatigue, weakness, shortness of breath, easy bruising or bleeding, frequent infections, fever, and unintentional weight loss. These symptoms arise from both the reduced number of healthy blood cells and the accumulation of abnormal cells in the marrow and bloodstream. AML can advance rapidly, making early diagnosis and treatment essential.
Find out more about acute myeloid leukemia drugs @ Acute Myeloid Leukemia Treatment
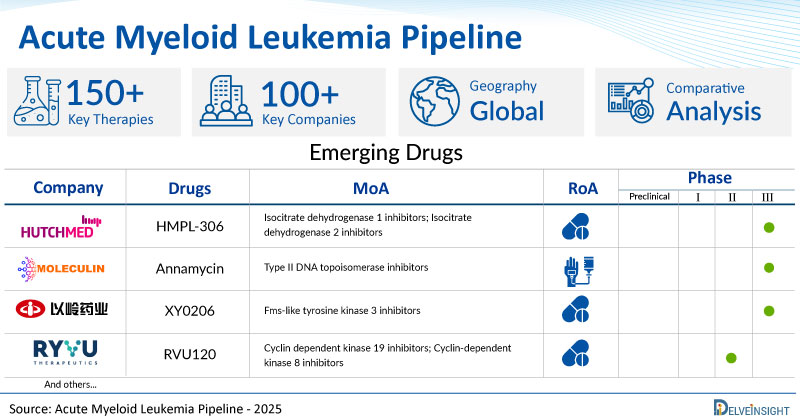
A snapshot of the Pipeline Acute Myeloid Leukemia Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| HMPL-306 | HUTCHMED | III | Isocitrate dehydrogenase 1 inhibitors; Isocitrate dehydrogenase 2 inhibitors | Oral |
| Annamycin | Moleculin Biotech, Inc. | III | Type II DNA topoisomerase inhibitors | Intravenous |
| XY0206 | Shijiazhuang Yiling Pharmaceutical Co. Ltd | III | Fms-like tyrosine kinase 3 inhibitors | Oral |
| RVU120 | Ryvu Therapeutics | II | Cyclin dependent kinase 19 inhibitors; Cyclin-dependent kinase 8 inhibitors | Oral |
| IO 202 | Immune-Onc Therapeutics | I/II | LILRB4 protein inhibitors | Intravenous |
| CTX 712 | Chordia Therapeutics, Inc. | I/II | CLK kinase inhibitors | Oral |
| APL 4098 | Apollo Therapeutics | I/II | GCN2 Inhibitor | Oral |
| HC-7366 | HiberCell, Inc. | I | GCN2 Modulator | Oral |
| CLN-049 | Cullinan Therapeutics Inc. | I | Antibody-dependent cell cytotoxicity | Intravenous |
Learn more about the acute myeloid leukemia treatment success rate @ Acute Myeloid Leukemia Clinical Trials
Recent Developments in Acute Myeloid Leukemia Treatment Space
- In November 2025, Cullinan Therapeutics announced new clinical data from its Phase I study of CLN-049 in patients with relapsed/refractory (r/r) acute myeloid leukemia (AML).
- In November 2025, Remedy Plan Therapeutics (RPT) announced that RPT1G was found to be safe and well-tolerated in a Phase I study in healthy volunteers at single and multiple ascending dose levels.
- In September 2025, Akeso Inc. announced that its proprietary next-generation humanized IgG4 monoclonal antibody targeting CD47, ligufalimab (AK117), has been granted Orphan Drug Designation (ODD) by the US FDA for the treatment of acute myeloid leukemia (AML).
- In June 2025, Senti Biosciences, Inc. announced that the US Food and Drug Administration (FDA) has granted Orphan Drug Designation to SENTI-202 for the treatment of relapsed/refractory hematologic malignancies including AML.
- In May 2025, Remedy Plan Therapeutics announced the company has raised over USD 18 million in an oversubscribed insider financing round. The financing will accelerate the advancement of the company’s first-in-class NAMPT inhibitor, RPT1G, into a Phase I/II clinical study in patients with AML.
- In May 2025, Moleculin Biotech, Inc., announced that the International Nonproprietary Names (INN) Expert Committee of the World Health Organization approved “naxtarubicin” for the non-proprietary name of the Company’s next-generation anthracycline in development, Annamycin for the treatment of relapsed or refractory acute myeloid leukemia (AML).
- In February 2025, TC BioPharm announced that it has concluded dosing of Cohort A patients in the ACHIEVE Phase IIB UK clinical trial that is an open-label Phase II study dedicated to evaluating the efficacy and safety of TCB008 in patients suffering from acute myeloid leukemia (AML).
- In October 2024, Advanced BioDesign announced the new design of the second part of its ODYSSEY clinical trial, evaluating ABD-3001, its first-in-class selective ALDH1A inhibitor in patients with relapsed/refractory (r/r) Acute Myeloid Leukemia (AML).
- In July 2024, Advesya announced that it received approval from the European Medicines Agency (EMA) to commence the Phase I/II trial of CCTx-001 for patients with acute myeloid leukemia.
- In June 2024, Advanced BioDesign released the first data from its first-in-human ODYSSEY study, aimed at treating acute myeloid leukemia (AML) with the drug candidate ABD-3001.
- In May 2024, HUTCHMED announced that it has initiated a registrational Phase III clinical trial of HMPL-306 in patients with mutated isocitrate dehydrogenase 1 or 2 relapsed / refractory acute myeloid leukemia in China.
Scope of the Acute Myeloid Leukemia Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
- Therapeutics Assessment By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy
- Therapeutics Assessment By Mechanism of Action: Isocitrate dehydrogenase 1 inhibitors; Isocitrate dehydrogenase 2 inhibitors, Type II DNA topoisomerase inhibitors, Fms-like tyrosine kinase 3 inhibitors, LILRB4 protein inhibitors, CLK kinase inhibitors, GCN2 Inhibitor, GCN2 Modulator, Antibody-dependent cell cytotoxicity, SNRNP200 protein modulators, Interleukin-1 receptor accessory protein inhibitors, CD47 antigen inhibitors
- Key Acute Myeloid Leukemia Companies: HUTCHMED, Senti Biosciences, Shijiazhuang Yiling Pharmaceutical Co. Ltd, Apollo Therapeutics, Immune-Onc Therapeutics, Molecular Partners, Kling Biotherapeutics, Chordia Therapeutics, Inc., Cullinan Therapeutics Inc., Moleculin Biotech, Inc., Advesya, AstraZeneca, Akeso, Galecto Biotech, TC Biopharm, Hangzhou Polymed Biopharmaceuticals, Inc., Ascentage Pharma, Ryvu Therapeutics, Guangzhou Bio-gene Technology Co., Ltd, CSPC ZhongQi Pharmaceutical Technology Co., Ltd., Remedy Plan, Inc., Advanced BioDesign, Ellipses Pharma, CCM Biosciences, HiberCell, Inc. and others.
- Key Acute Myeloid Leukemia Pipeline Therapies: HMPL-306, SENTI 202, XY0206, APL 4098, IO 202, MP0533, KBA 1331, CTX-712, CLN-049, Annamycin, CCTx-001, AZD3632, AK117, GB3226, TCB008, HPB-092, APG-2575, RVU120, BG1805, SYHX1903, RPT1G, ABD-3001, EP0042, CCM-445, HC-7366 and others.
Dive deep into rich insights for new acute myeloid leukemia therapy, visit @ Acute Myeloid Leukemia Treatment Medicine
Table of Contents
| 1. | Acute Myeloid Leukemia Pipeline Report Introduction |
| 2. | Acute Myeloid Leukemia Pipeline Report Executive Summary |
| 3. | Acute Myeloid Leukemia Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Acute Myeloid Leukemia Clinical Trial Therapeutics |
| 6. | Acute Myeloid Leukemia Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Acute Myeloid Leukemia Pipeline: Late-Stage Products (Phase III) |
| 8. | Acute Myeloid Leukemia Pipeline: Mid-Stage Products (Phase II) |
| 9. | Acute Myeloid Leukemia Pipeline: Early-Stage Products (Phase I) |
| 10. | Acute Myeloid Leukemia Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Acute Myeloid Leukemia Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Acute Myeloid Leukemia Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the acute myeloid leukemia medications, reach out @ Acute Myeloid Leukemia Treatment Drugs
Related Reports
Acute Myeloid Leukemia Epidemiology Forecast
Acute Myeloid Leukemia Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted acute myeloid leukemia epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Acute Myeloid Leukemia Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key AML companies, including CicloMed LLC, Jazz Pharmaceuticals, Minneamrita Therapeutics LLC, Syndax Pharmaceuticals, Astex Pharmaceuticals, Inc., Karyopharm Therapeutics Inc, Sanofi, Polaris Group, Bio-Path Holdings, Inc., Chordia Therapeutics, Inc., Theradex, BioTheryX, Inc., Precigen, Inc, Eli Lilly and Company, Bayer, Takeda, Meryx, Inc., 2seventy bio, JW Pharmaceutical, Telios Pharma, Inc., Kartos Therapeutics, Inc., Celyad Oncology SA, Merck Sharp & Dohme LLC, Celgene, AbbVie, Genentech, Wugen, Inc., Arcellx, Inc, NextCure, Inc., Bellicum Pharmaceuticals, ImmunoGen, Inc., Astellas Pharma Inc, Aptose Biosciences Inc, Ascentage Pharma Group Inc., BioSight Ltd., GlycoMimetics Incorporated, Gilead Sciences, Chimerix, Daiichi Sankyo, Ryvu Therapeutics SA, Syros Pharmaceuticals, PrECOG, LLC, Cleave Therapeutics, Inc., Kronos Bio, Cullinan Oncology, LLC, Actinium Pharmaceuticals, Amgen, In8bio Inc, Sellas Life Sciences Group, Kura Oncology, Inc., Arog Pharmaceuticals, Inc., Novo Nordisk A/S, Marker Therapeutics, Inc., Shattuck Labs, Inc., Oncoceutics, Inc., among others.
Relapsed/Refractory Acute Myeloid Leukemia Market
Relapsed/Refractory Acute Myeloid Leukemia Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key R/R AML companies, including Ascentage Pharma, Cullinan Oncology, Kronos Bio, Maxinovel Pharmaceuticals, AB Science, CicloMed, Karyopharm Therapeutics, Antengene Corporation, GlycoMimetics, Servier, Novartis, ImmunoGen, among others.
Chronic Lymphocytic Leukemia Market
Chronic Lymphocytic Leukemia Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key chronic lymphocytic leukemia companies, including Loxo Oncology, Oncternal Therapeutics, MingSight Pharmaceuticals, Nurix Therapeutics, Starton Therapeutics, TG Therapeutics, Bristol Myers Squibb, Novartis, Aprea Therapeutics, AstraZeneca, Genor Biopharma, Incyte Corporation, MorphoSys, Astex Therapeutics, Lava Therapeutics, Celgene Corporation, among others.
Chronic Myeloid Leukemia Market
Chronic Myeloid Leukemia Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key chronic myeloid leukemia companies, including Salarius Pharmaceuticals, Jazz Pharmaceuticals and Pharmamar, Eli Lilly, Pfizer, Bioatla, Cellectar Biosciences, Sumitomo Pharma Oncology, Inhibrx, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
